Open here for our page navigation
Mercury
99.99 - 99.9999% pure
Mercury is the only common metal which is liquid at ordinary temperatures. It is a heavy, silvery-white liquid metal.
It is a rather poor conductor of heat as compared with other metals but it is a conductor of electricity.
It alloys easily with many metals, such as gold, silver, zinc, cadmium and tin. These alloys are called amalgams.
Mercury metal has many uses. Because of its high density, it is used in barometers and manometers. It is extensively used in thermometers, because of its’ high rate of thermal expansion which is fairly constant over a wide temperature range.
Mercury is also used in a variety of chemistry and physics experiments.
Industry uses mercury metal as a liquid electrode in the manufacture of chlorine and sodium hydroxide by electrolysis of brine.
Mercury is still used in some electrical gear, such as switches and rectifiers, which need to be reliable, and for industrial catalysis. Much less mercury is now used in consumer batteries and fluorescent lighting, but it has not been entirely eliminated. Other industrial uses include sealed electrical discharge tubes and vacuum apparatus.
Specifications and Properties:
Formula: Hg
CAS Number: 7439-97-6
Molecular Weight: 200.59
Appearance: Silver-white liquid
Density: 13.534 gm/ml
Melting Point: -38.87oC
Boiling Point: 356.6oC
Resistivity: 95.8 μΩ-cm @ 20°C
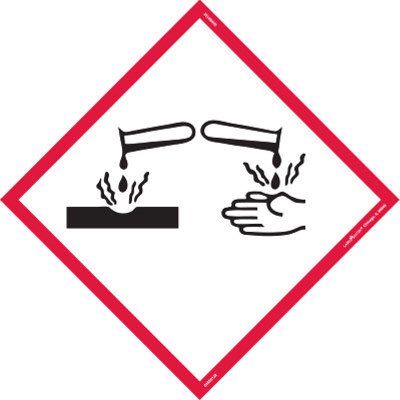
Mercury is a very toxic element. It can enter the body through an open wound or by inhaling or ingesting it. It can then cause damage to nerves, the liver and the kidney, as well as a number of other symptoms.
Mercury is a Hazard for Shipping: UN 2809, Class 8, PG III